Project Overview
Making Neurotechnology Accessible to All
Addressing Inequities and Adoption Disparities in Neurotechnology among Marginalised Groups.
This project aims to promote the equitable development and adoption of neurotechnologies by identifying and addressing biases, barriers, and disparities in their design, performance, and utilisation among marginalised groups. By employing user-centred approaches and community-based research that integrate both qualitative and quantitative analyses, we will achieve this aim through the following objectives:
- Identify biases and inequities in the design and performance of neurotechnologies.
- Understand barriers to the participation of minority ethnic groups in neuroscience and neurotechnology research.
- Investigate racial and gender disparity in the utilisation of neurotechnologies.
- Co-develop practical solutions, guidelines and recommendations to address these challenges.
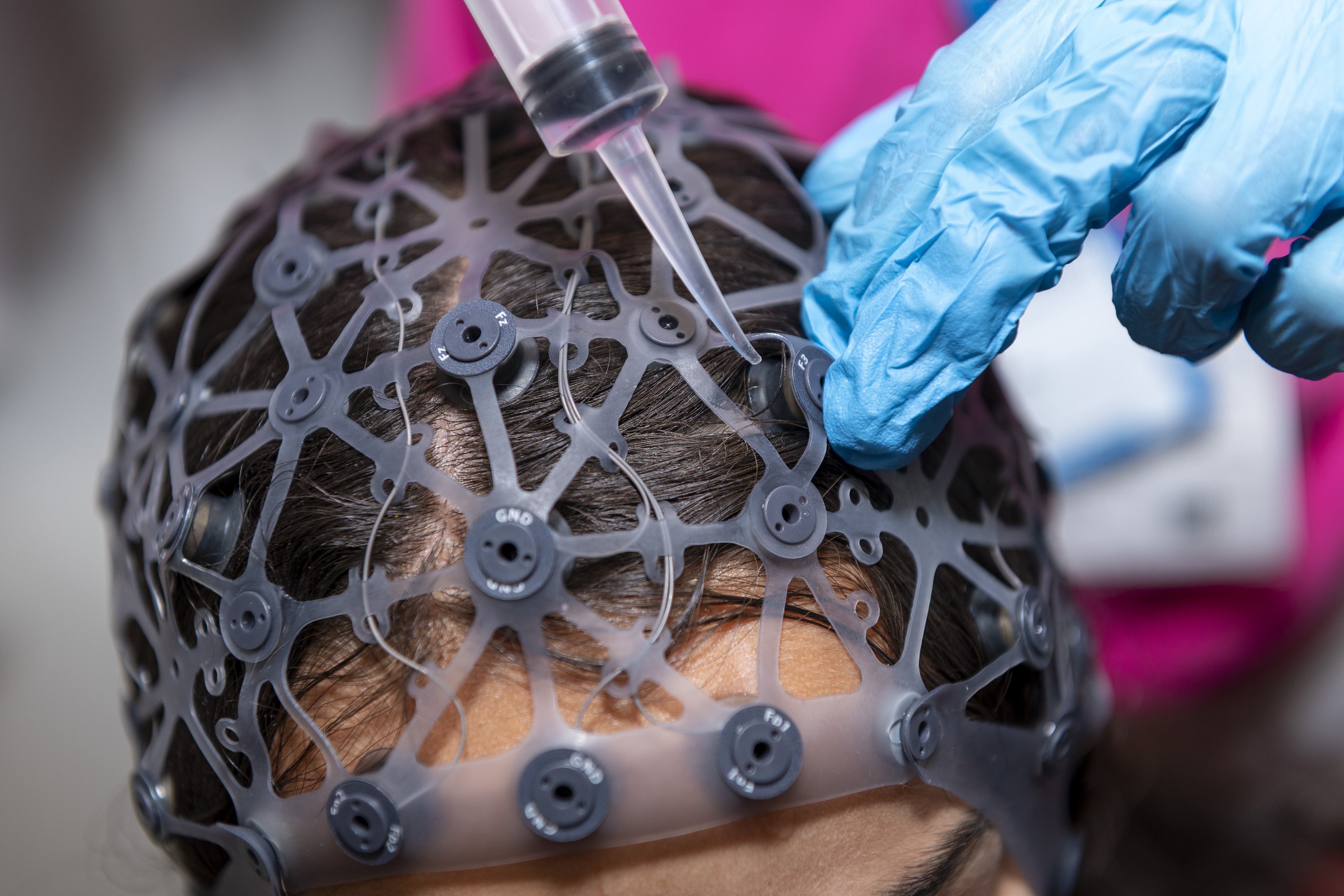
Calibration and Design Biases
Neurotechnology biases refer to systematic discrepancies that result in unequal outcomes or access for different demographic groups. Calibration and design biases are prominent, with technologies like fNIRS[1] and EEG[2] often not calibrated for diverse skin tones and hair types, leading to inaccurate readings and poor performance for people with darker skin or thick, coarse hair. Additionally, variations in bone density, skull shape and thickness across different ethnic groups and genders can impact the effectiveness of neurotechnologies[3], like focused ultrasound neurostimulation, and transcranial magnetic stimulation (TMS), if not calibrated appropriately.
We will identify biases and performance discrepancies across current and emerging neurotechnologies by combining expert interviews and analysis of existing datasets. The focus is on how inadequate design and calibration of software and hardware can disadvantage users with different skin tones, skull anatomies, bone densities, hairstyles, and genetic or hormonal profiles.
The emerging technologies examined will span electrical, optical, acoustic, chemical, thermal, and magnetic methods for recording and/or stimulating brain activity, both invasive and non-invasive. Findings will feed into practical recommendations so these systems are accessible and effective for all users.
- Map known biases and performance gaps
- Quantify anatomical and tissue variability across groups using existing datasets
- Estimate performance loss from non-calibrated use in FUS, tDCS and related modalities
- Compare calibration strategies for software and hardware across skin tones, hair types, and skull properties
Outputs. Evidence map of biases, annotated parameter ranges for anatomy and tissue, modelling scripts and benchmarks, and actionable calibration and design guidance for labs, clinics, and vendors.
What success looks like. Reduced groupwise performance gaps in simulations and benchtop tests, clearer calibration guidance adopted in protocols, and improved reliability across diverse users in subsequent studies.

Inclusive Participation in Research
Participation is not evenly distributed. In one industry dataset of global neuroscience trials, only 1.6% of 8,015 participants were Black while 85.6% were White[4]. In the UK Biobank, about 95% of neuroimaging data is from White participants[5,6]. If these disparities in clinical trial participation are not addressed urgently, they could significantly hinder the adoption, inclusivity, and acceptance of neurotechnology among minority populations.
We conduct community-based participatory research to understand barriers to participation in neuroscience and neurotechnology research. Through semi-structured interviews and focus groups with non-white minority communities, we will explore cultural, religious, socioeconomic, language and education factors, as well as stigma and mistrust toward brain research.
- Co-develop case studies with Community Research Champions; run focus groups and interviews in trusted community settings
- Identify practical enablers (translation, childcare, transport, flexible timing) and cultural considerations that shape participation
- Use iterative review cycles with participants, researchers and clinicians to refine barriers and solutions
- Produce clear guidance that teams can adopt in recruitment, consent and study conduct
Outputs. A concise participation toolkit, plain-language materials, recruitment and consent templates, and a barriers-solutions evidence summary for researchers and sponsors.
What success looks like. More representative research participation and stronger trust indicators across participating communities.

Equitable Access and Use
Recent evidence shows significant underrepresentation of women in clinical trials and a lower likelihood of women undergoing deep brain stimulation (DBS) compared to men[7,8]. Racial and ethnic minorities also face lower rates of DBS procedures, even when accounting for disease severity and other demographic factors[8,9]. For Parkinson's disease, one study found White patients were about five times more likely to receive DBS than Black patients[9]. A multi-center review in Germany and England found women were less likely to be referred and to accept DBS despite similar benefits[10].
Addressing these disparities, both from the patient perspective and in clinical decision-making, is crucial for developing equitable neurotechnology solutions, including DBS and future neural interfaces for treating neurological conditions.
- Quantify disparities across the DBS pathway, stratified by gender, ethnicity and deprivation
- Build a reproducible analysis using Data Connect records linking symptoms, comorbidities and outcomes
- Co-develop interview protocols with patient groups; conduct and thematically code interviews with patients and clinicians to surface barriers and enablers
Outputs. DBS equity insights summary, and a set of interventions and recommendations.
What success looks like. Smaller gaps in referral, offer and uptake by gender and ethnicity, better patient understanding and satisfaction, and adoption of equity prompts and dashboards by participating centres.
References
10- Kwasa, J., et al. (2023). Demographic reporting and phenotypic exclusion in fNIRS. Frontiers in Neuroscience. 10.3389/fnins.2023.1331375. ↩︎
- Penner, F., et al. (2023). Racial disparities in EEG research and their implications for our understanding of the maternal brain. Cognitive, Affective, and Behavioral Neuroscience. 10.3758/s13415-022-01040-w. ↩︎
- Zhang, J., et al. (2023). Automatic analysis of skull thickness, scalp-to-cortex distance and association with age and sex in cognitively normal elderly. Brain Stimulation. 10.1101/2023.01.19.524484. ↩︎
- Rutten-Jacobs, L., et al. (2024). Racial and ethnic diversity in global neuroscience clinical trials. Contemporary Clinical Trials Communications, 37, 101255. 10.1016/j.conctc.2024.101255. ↩︎
- Fry, A., et al. (2017). Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. American Journal of Epidemiology, 186, 1026-1034. 10.1093/aje/kwx246. ↩︎
- Ricard, J. A., et al. (2023). Confronting racially exclusionary practices in the acquisition and analyses of neuroimaging data. Nature Neuroscience, 26, 4-11. 10.1038/s41593-022-01218-y. ↩︎
- Kübler, D., et al. (2023). Gender-specific outcomes of deep brain stimulation for Parkinson's disease. Neurological Sciences, 44, 1625-1631. 10.1007/s10072-023-06598-y. ↩︎
- Hughes, N. C., Bishay, A. E., et al. (2024). Disparities in access to deep brain stimulation for Parkinson's disease and proposed interventions: A literature review. Stereotactic and Functional Neurosurgery, 102(3), 179-194. 10.1159/000538748. ↩︎
- Cramer, S. W., et al. (2022). Persistent racial disparities in deep brain stimulation for Parkinson's disease. Annals of Neurology, 92(2), 246-254. 10.1002/ana.26378. ↩︎
- Jost, S. T., et al. (2022). Gender gap in deep brain stimulation for Parkinson's disease. npj Parkinson's Disease, 8, 47. 10.1038/s41531-022-00305-y. ↩︎